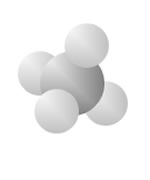
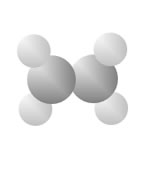
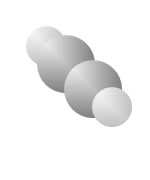
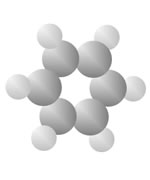
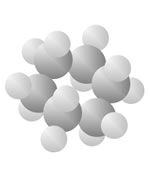
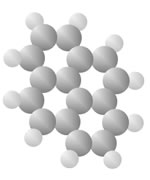
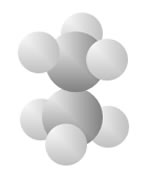
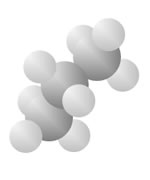
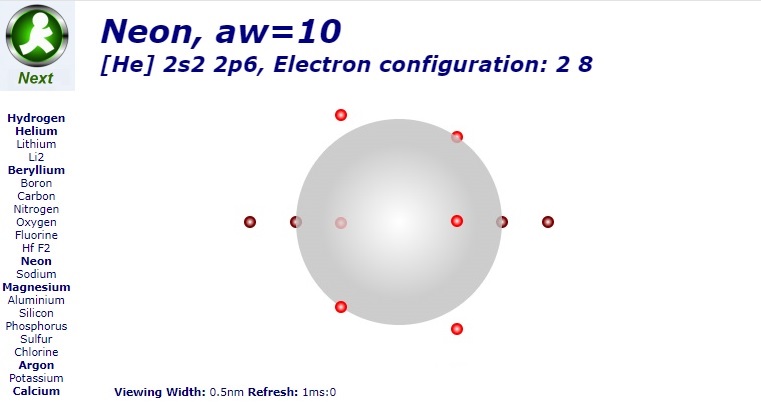
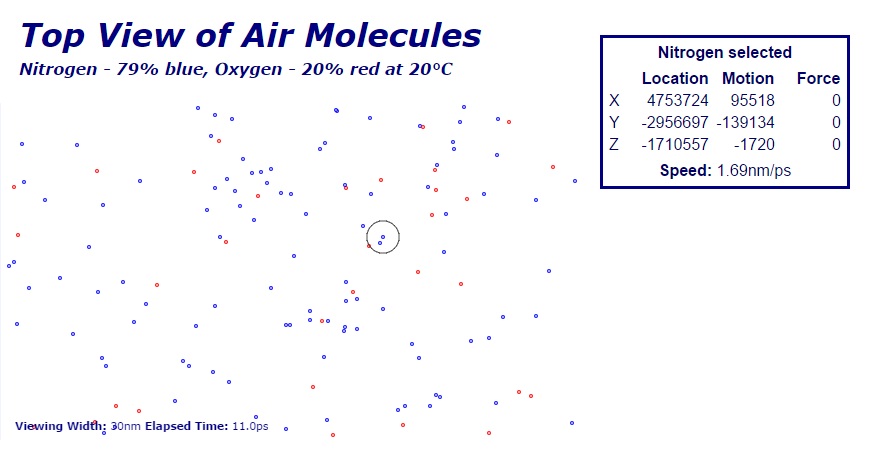
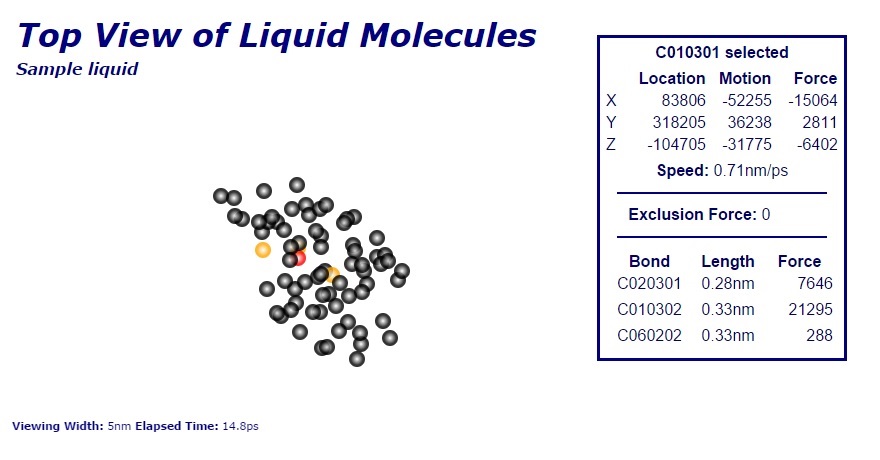
Hydrocarbons
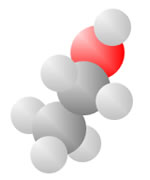
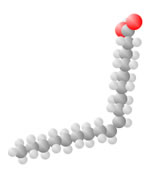
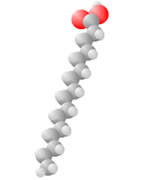
Hydrocarbons consist of carbon (gray) and hydrogen (silver) elements with different relative concentrations accounting for different forms.
Alcohols and Sugars
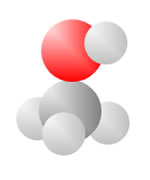
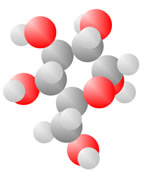
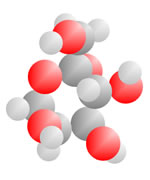
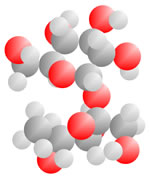
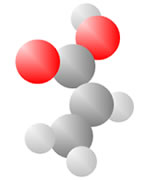
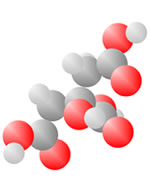
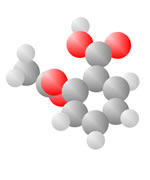
Adding an oxygen (red) with only one bond available produces an Alcohol. An oxygen (red) with two bonds in a carbon loop produce Sugars.
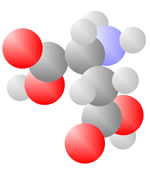
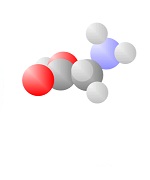
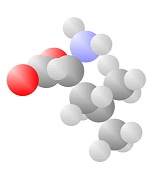
Acids
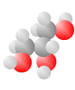
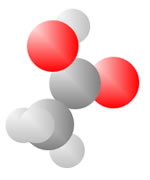
Acids come to be with both the single bond oxygen (red) and the double bond oxygen (red) on the same carbon.
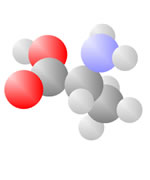
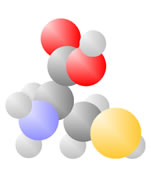
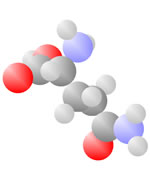
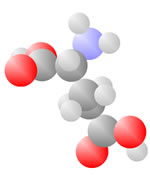
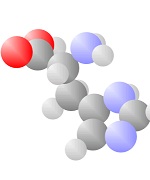
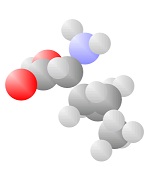
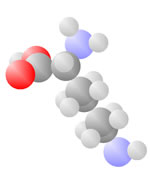
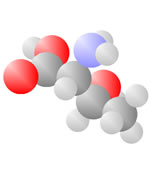
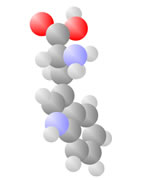
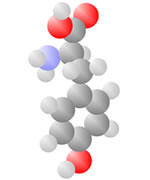
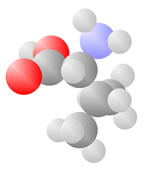
Amino Acids
Adding a carbon and a nitrogen (blue) to the acid produces the Amino Acids, the basic ingredients to life.
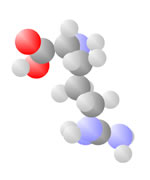
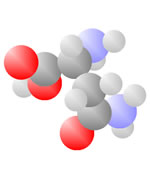
And More