Electron Bonding
The electron is a highly charged magnet. Electrons repel each other due to the coulomb force. Electrons also attract each other due to their magnetic nature. The north pole of one electron will be attracted to the south pole of another electron.
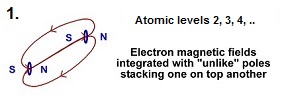
Electrons trapped in particular arrangements due to the location of other electrons and protons around them will tend to magnetically "bind" to each other. Electrons can bind to each other in a number of different ways. Being magnetic, they can stack on top of each other with the north pole of one electron, pointing to the south pole of another electron. The electron is modelled as a spinning magnetic field held captive by an orthogonal spinning electrical field. In the case of "bound" electrons, not only is the magnetic force drawing them together, but they are also bonded through the integration of their own magnetic force lines.
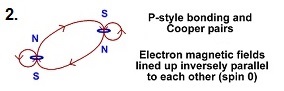 Electrons can also bind by lining up side by side with
the north pole of one electron pointing in the same direction as the south pole
of another electron. These electrons also integrate their internal magnetic structure
and become bound. The bound electrons only get as close to each other as
the surrounding coulomb forces will allow (ie. the electrons will repel each other
through the squared distance inverse force law).
Electrons can also bind by lining up side by side with
the north pole of one electron pointing in the same direction as the south pole
of another electron. These electrons also integrate their internal magnetic structure
and become bound. The bound electrons only get as close to each other as
the surrounding coulomb forces will allow (ie. the electrons will repel each other
through the squared distance inverse force law).
It is easy to detect which type of bonding electrons have done by their spin. Normally, an electron is a spin 1/2 particle. That means that if you shoot a beam of electrons through a magnetic field, 1/2 of the electrons will go up in the magnetic field, and the other half will go down. None of the electrons will go "straight" in a magnetic field. Electrons bound as in figure1 are still spin 1/2 particles. They still will only go up or down in a magnetic field. On the other hand, electrons bound as in figure2 are spin 0 particles. This means that a magnetic field has no effect on them at all. If you shoot these electron pairs through a magnetic field, the force on one electron is directly opposite to the force on the second electron. The result is the electrons paired in this manner are not affected by a magnetic field and will go "straight" through the magnetic field.
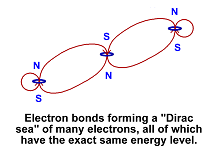
Electrons can form multiple bounds to each other as with proper north/south combinations. This accounts for the different style of bonding between molcules where sometimes a bond depends on 2, 3 or even 4 bonded electrons. In a metal, free electrons can bind together to form "Cooper pairs" or an entire group of electrons can form a "Dirac sea" of electrons, all at the exact same energy level.
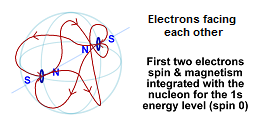
Electrons can also integrate their magnetic field and spin with a proton. This involves a much lower level of energy and is called the "Lamb Shift". In this case, the electrons end up facing each other (a spin 0 particle) and the magnetic field is not only integrated with the other electron at this energy level, as well as the protons magnetic field.