Simple Solids
Explore solids by clicking on the picture of the solid. You will enter a 3D perspective view from just above the surface of the solid. Drag a mouse across the screen to move forward, back or to turn. If touchscreen, drag your finger. You can see an overhead or topdown view of yourself in the top right-hand window.
Carbon and Silicon
The properties of carbon vary with the form. Diamond is transparent and hard, graphite is black and soft. Diamond has low conductivity, while graphite is a very good conductor.
Silicon crystallizes in a diamond cubic crystal structure, with a lattice spacing of approximately 0.54nm. The outer electron orbital of silicon has four valence electrons available for binding.
Copper
Copper has one s-orbital electron on top of a filled d-electron shell and is characterized by high ductility and electrical conductivity. Interatomic interactions are dominated by the s-electrons through metallic, not covalent bonds.
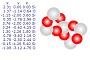
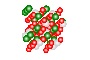
Quartz Crystal
Silicon and Oxygen atoms

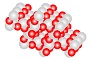
Quartz is found in the Earth's continental crust. It is made up of continuous SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz, several of which are semi-precious gemstones.
Quartz crystals have piezoelectric properties: they develop an electric potential upon the application of mechanical stress. An early use of this property of quartz crystals was in phonograph pickups. A common piezoelectric use of quartz today is as a crystal oscillator. The quartz clock is a familiar device using the mineral. The resonant frequency of a quartz crystal oscillator is changed by mechanically loading it, and this principle is used for very accurate measurements of very small mass changes in the quartz crystal microbalance and in thin-film thickness monitors.
Crystallography of Quartz
(Mineral data from: http://www.mindat.org/min-3337.html)
- System: Trigonal, Class (H-M): 3 2 - Trapezohedral, Space Group: P31 2 1
- Parameters: a = 4.9Å, c = 5.4Å, Ratio: a:c = 1 : 1.1, Unit Cell Volume: V 113.00 ų
- Morphology: Typically long prismatic with steep pyramidal terminations, but may be short prismatic to bipyramidal, or needle-like; massive material (especially agate & chalcedony) may be microscopically fibrous.
Additional
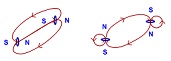
Exotic material with respect to magnetic, superconducting and photonic properties
Found on Mars, December, 2012
Clays: The silicate minerals make up the largest and most important class of rock-forming minerals, constituting approximately 90 percent of the crust of the Earth. They are classified based on the structure of their silicate group. Silicate minerals all contain silicon and oxygen.