The Bohr Radius - 5.29x10⁻⁹ meters
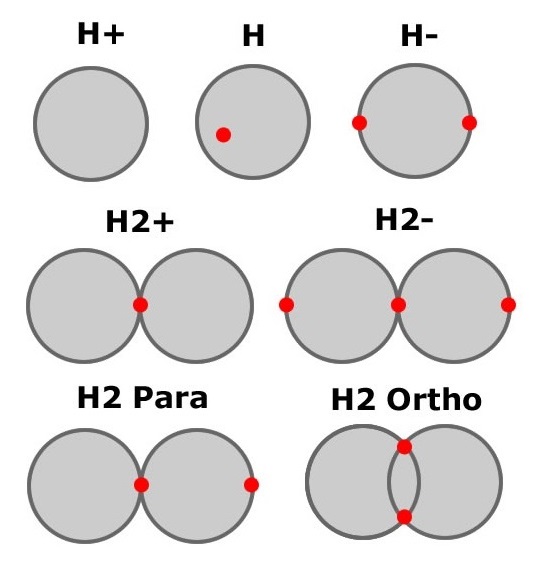 The ionization energy of the hydrogen atom is 13.6 eVolts. This is the amount of energy required to completely separate the
electron from the proton. If you stand on earth, there is a certain amount of energy that is required to blast
you out of earth orbit and send you to the moon or mars. 13.6 eVolts is the amount of energy required to separate
an electron and proton starting from a distance of 52.9pm. It is interesting that you never need more then 13.6 eVolts
to separate the electron and proton.
The ionization energy of the hydrogen atom is 13.6 eVolts. This is the amount of energy required to completely separate the
electron from the proton. If you stand on earth, there is a certain amount of energy that is required to blast
you out of earth orbit and send you to the moon or mars. 13.6 eVolts is the amount of energy required to separate
an electron and proton starting from a distance of 52.9pm. It is interesting that you never need more then 13.6 eVolts
to separate the electron and proton.
To model this, we assume the charge is distributed on the surface of the shell. This means the electron feels no force from the proton when inside the 52.9pm shell proton shell, but normal coulomb force outside the shell. Have fun playing the game "Shoot the Electron" and try ionizing various forms of hydrogen yourself.
Energy Levels and Shells
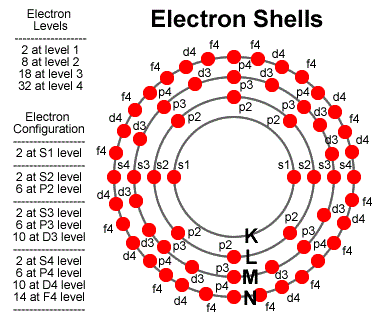 The energy levels of an atom act within groups where certain numbers of electrons are allowed within each shell.
Each shell, is further subdivided by the type of oscillations that can make up the shell. An s-level shell only oscillates in one pattern
so supports only two electrons, one on each side. A p-level shell oscillates in 3 different patterns and can support 6 electrons. Similarily,
a d-level shell can support 10 electrons and so on.
The energy levels of an atom act within groups where certain numbers of electrons are allowed within each shell.
Each shell, is further subdivided by the type of oscillations that can make up the shell. An s-level shell only oscillates in one pattern
so supports only two electrons, one on each side. A p-level shell oscillates in 3 different patterns and can support 6 electrons. Similarily,
a d-level shell can support 10 electrons and so on.
To model this, we assume the electron becomes attached to a specific shell and oscillates based on its energy. The electron energy determines its wave function and the wave function must resonate with the proton wave function. An n=2 level shell is simply the n=1 shell oscillating at a higher resonant frequency. An electron will typically fall into a high level shell where it vibrates in a pretty unstable pattern at say level f8 or 9. Over time the electron stablizes and falls to a p2 or 3, eventually settling in as an s1 state. Each movement emits a photon of energy reflecting the differences in energy of the start versus the ending energy level.


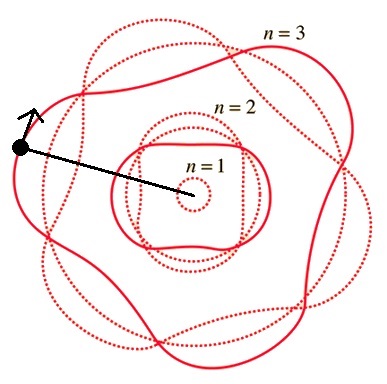 Quantum Mechanics modelling of energy levels uses a rigid rotator. The path of the electron around the proton must
equal an integer number of waves.
Unfortunately, the visual representation of a rigid rotator is the electron flying around the proton
falls apart if you try to visualize 100's of electrons in a molecule or part of a solid. There is a famous quote often
used to explain this, "shut up and calculate".
Quantum Mechanics modelling of energy levels uses a rigid rotator. The path of the electron around the proton must
equal an integer number of waves.
Unfortunately, the visual representation of a rigid rotator is the electron flying around the proton
falls apart if you try to visualize 100's of electrons in a molecule or part of a solid. There is a famous quote often
used to explain this, "shut up and calculate".
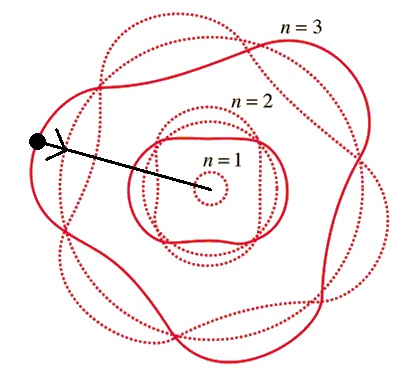 Animated Physics uses the same calculation, but illustrates it differently. The wave function of the oscillating electron resonates
with the wave function of the proton shell where where the wave function of the electron must be an integer multiple
of the wave function of the proton shell. Now we can model the electron at a specific location, held there with specific
amounts of energy.
Animated Physics uses the same calculation, but illustrates it differently. The wave function of the oscillating electron resonates
with the wave function of the proton shell where where the wave function of the electron must be an integer multiple
of the wave function of the proton shell. Now we can model the electron at a specific location, held there with specific
amounts of energy.